Gene Expression Lab
Sean Chien
2022-11-05
Group of 4
2 people do lab activity 1; 2 people do lab activity 2
Lab Activity 1: Determine the optimal growth conditions for LacZ expression.
| Culture Conditions | Rxn # | Lysis Reagent | E. coli Culture Volume | TSB | Z Buffer | ONPG |
|---|---|---|---|---|---|---|
| No suagr E. coli culture | 1a | 10 ul | 10 ul | 90 ul | 690 ul | 200 ul |
| 1b | 10 ul | 20ul | 80 ul | 690 ul | 200 ul | |
| 1c | 10 ul | 100 ul | 0 ul | 690 ul | 200 ul | |
| Lactose E. coli culture | 2a | 10 ul | 10 ul | 90 ul | 690 ul | 200 ul |
| 2b | 10 ul | 20ul | 80 ul | 690 ul | 200 ul | |
| 2c | 10 ul | 100 ul | 0 ul | 690 ul | 200 ul | |
| Glucose E coli culture | 3a | 10 ul | 10 ul | 90 ul | 690 ul | 200 ul |
| 3b | 10 ul | 20ul | 80 ul | 690 ul | 200 ul | |
| 3c | 10 ul | 100 ul | 0 ul | 690 ul | 200 ul | |
| Glucose + Lactose E coli culture | 4a | 10 ul | 10 ul | 90 ul | 690 ul | 200 ul |
| 4b | 10 ul | 20ul | 80 ul | 690 ul | 200 ul | |
| 4c | 10 ul | 100 ul | 0 ul | 690 ul | 200 ul | |
| Blank | Bla | 10 ul | 0 | 100 | 690 ul | 200 ul |
Dilute the E. coli culture 1:5 by pipetting 100 µL of E. coli culture into a microcentrifuge tube. Add 400 µL of distilled water, close the cap, and mix well.
(You should dilute 4 culture conditions, respectively)
- Clearly label 13 microcentrifuge tubes to correspond with the
reactions listed in Table 1(Rxn #).
- Pipet the appropriate E. coli culture, TSB, and Lysis Reagent into 13 labelled tubes. (adding Lysis Reagent last)
- Cap the tubes and mix by gently flicking with your finger. Incubate the tubes at room temperature for 10 min.
While the E. coli is lysing, clearly label 13 plastic cuvette tubes to correspond with the reactions in Table 1 (Rxn #).
(labelling up near the top of the tube so it will not interfere with absorbance readings. )Pipet the appropriate volume of Z Buffer into each of the 13 plastic cuvette tubes and the blank as calculated in Table 1.
When the E. coli are done lysing, place the microcentrifuge tubes on ice.
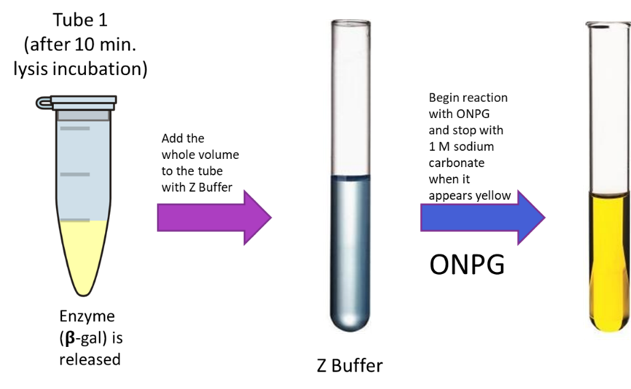
Quantitatively transfer the contents of each of your lysis microcentrifuge tubes to the corresponding plastic cuvette tubes containing the Z buffer.
- What setting should you need on your micropipettor to do this? 110ul
Add the volume of ONPG stock solution in Table 1 to each reaction
- flick gently to mix, and incubate at room temperature to begin the reactions.
- Set a timer to count up and record the time when ONPG was added to each reaction tube in Table 2.
When the reaction turns yellow (about the color of a Post-It note), stop the reaction by adding 500 µL of 1M sodium carbonate and record the time you stopped the reaction in Table 2.
Read the absorbance at A420nm and record in Table 2.
- Be sure to gently wipe off the cuvette with a Kimwipe before inserting the cuvette into the instrument.
Calculate β-Gal activity (record in Table 2).
Lab Activity 2: What are the effects of adding sugars to the β-galactosidase assay?
- Important considerations: once cultures are mixed with sugar in step 2, the conditions have changed, so gene expression will start to change. Therefore, be prepared (tubes labeled and organized, etc.) to immediately begin lysing the cells. You might consider measuring out all of your culture materials first, and then going back and adding the lactose or glucose. It should take less than 10 minutes from preparing the first culture in step 2 to beginning the lysis.
- Clearly label 8 microcentrifuge tubes to correspond with the
reactions listed in Table 3 (two tubes per culture condition).
- 1st microcentrifuge tubes for each reaction (Tube 1), pipet the
TSB media and lysis
reagent
- DO NOT add Z Buffer and ONPG yet. They are added later in cuvettes
- 2nd microcentrifuge tube (Tube 2), pipet 475 µL of the appropriate culture and 25 µL of the corresponding sugar
- 1st microcentrifuge tubes for each reaction (Tube 1), pipet the
TSB media and lysis
reagent
| Culture Conditions | Rxn # | TSB | Lysis Reagent | 20% Lactose | 20% Glucose | E. coli culture | E. coli volume from 2nd tube | Z Buffer | ONPG |
|---|---|---|---|---|---|---|---|---|---|
| No suagr E. coli culture + 20% Lactose | a-1 | 40 ul | 5 ul | / | / | / | 10 ul | 745 ul | 200 ul |
| a-2 | / | / | 25 ul | 0 ul | 475 ul | ||||
| Glucose E. coli culture + 20% Lactose | b-1 | 40 ul | 5 ul | / | / | / | 10 ul | 745 ul | 200 ul |
| b-2 | / | / | 25 ul | 0 ul | 475 ul | ||||
| No sugar E coli culture + 20% Glucose | c-1 | 40 ul | 5 ul | / | / | / | 10 ul | 745 ul | 200 ul |
| c-2 | / | / | 0 ul | 25 ul | 475 ul | ||||
| Lactose E coli culture + 20% Glucose | d-1 | 40 ul | 5 ul | / | / | / | 10 ul | 745 ul | 200 ul |
| d-2 | / | / | 0 ul | 25 ul | 475 ul | ||||
| Blank |
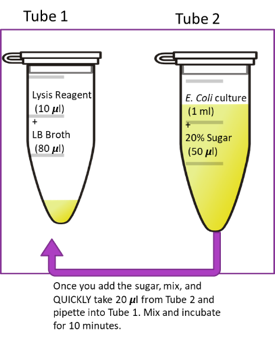
Pipet 10 uL of each culture/sugar mixture (2nd tube)into each microcentrifuge tube containing the unused culture media and lysis Reagent (1st)
10 ul tube-2 > tube-1
(Cap the tubes and mix by gentle flicking with your finger. Allow the bacteria to lyse by incubating the microcentrifuge tubes at room temperature for 10 min.)While the E. coli is lysing, clearly label 4 plastic cuvette tubes to correspond with the reactions in Table 3.2.(labelling up near the top of the tube so it will not interfere with absorbance readings.)
Pipet the appropriate volume of Z Buffer into each of the 4 plastic cuvette tubes as calculated in Table 3.
When the E. coli are done lysing, place the microcentrifuge tubes on ice.
Quantitatively transfer the contents of each of your lysis reactions from the microcentrifuge tubes to the corresponding plastic cuvette tubes containing the Z buffer.
Add the volume of ONPG stock solution in Table 3 to each reaction
- flick gently to mix, and incubate at room temperature to begin the reactions.
- Set a timer to count up and record the time when ONPG was added to each reaction tube in Table 4.
When the reaction turns yellow (about the color of a Post-It note), stop the reaction by adding 500 µL of 1M sodium carbonate and record the time you stopped the reaction in Table 4.
Read the absorbance at A420nm and record in Table 4.
- Be sure to gently wipe off the cuvette with a Kimwipe before inserting the cuvette into the instrument.
Calculate β-Gal activity (record in Table 4).
Post-Lab
Graph looks like…